Certified quality
Legislative Information
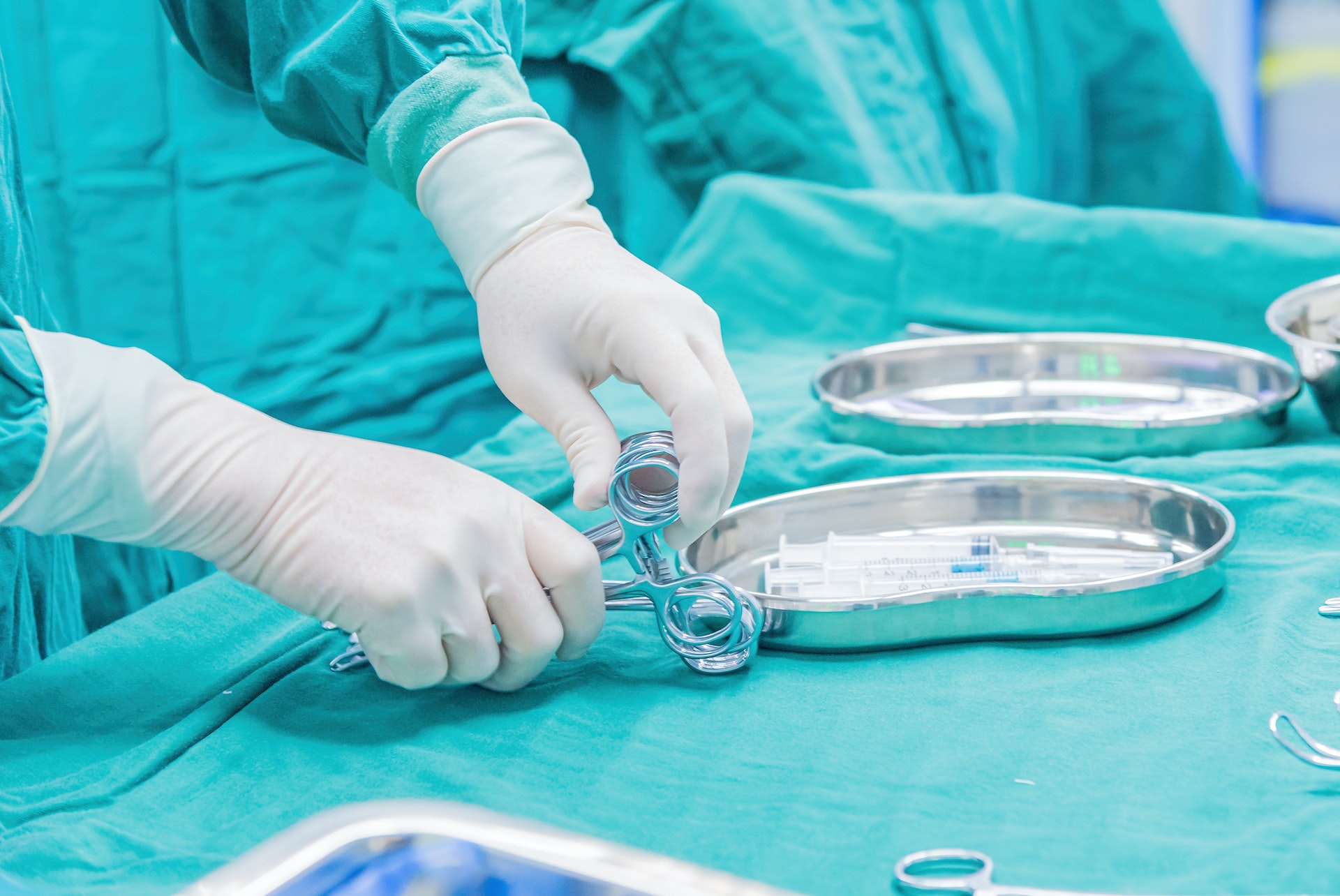
The prerequisite for placing disposable gloves on the market in the European Economic Area is confirmation of compliance with all the
applicable EU requirements by means of CE marking. Protective gloves, as part of personal protective equipment, protect the user’s hands
and, if necessary, the forearms of the user from external influences.
It protects patients
and health care professionals from contamination. The double declaration of a disposable glove, both as a medical device and a
and as a product of personal protective equipment, is possible. The guidelines form the basis for the classification of the various
products into categories or classes.